Determination of gas exchange in plant leaves by LI-6400 portable photosynthesizer
LI-6400 portable photosynthesis instrument to determine the gas exchange of plant leaves is to master the infrared CO2 analyzer method to determine the gas exchange parameters (photosynthetic rate, dark respiration, transpiration rate and stomatal conductance) determination of the basic principles; master the LI-6400 portable photosynthetic system to determine the photosynthetic rate, dark respiration, transpiration rate and stomatal conductance method and the determination of photosynthesis-light response curve The method of photosynthesis-light response curve.
Principle
How the Infrared CO2 Gas Analyzer (IRGA) works.
Infrared is an electromagnetic wave with a wavelength in the range of 0.75~400 μm. Infrared according to its wavelength, 25 ~ 400 μm for the far infrared; 2.5 ~ 25 μm for the mid-infrared; 0.75 ~ 2.5 μm for the near infrared. The heated object is an excellent source of infrared radiation. Infrared radiation in the propagation of its radiant energy is absorbed by the object is easy to detect, this feature has become the basis for the design and manufacture of infrared CO2 gas analyzers. Different gases absorb infrared radiation differently. Gas molecules composed of the same kind of atoms (such as N2, H2, O2, etc. ) do not absorb infrared. Only gas molecules composed of heterogeneous atoms (such as CO, CO2, CH2, H2O, etc.) can absorb infrared light. Because the gas molecules composed of heterogeneous atoms are permanently polarized molecules, i.e. dipoles. The positions of the atoms within the molecule are in constant motion and undergo periodic changes. Under the influence of infrared radiation of the same frequency, the dipoles (e.g. CO2, CH4) will resonate and absorb infrared radiation energy.
When CO2 gas absorbs infrared radiation energy, its molecular structure changes from symmetrical to telescopic or curved. In addition, CO2 gas can absorb infrared energy in four bands, and the wavelengths of the absorption peaks are 2.66 μm, 2.77 μm, 4.26 μm, and 14.99 μm, and the absorption rates are 0.54%, 0.31%, 23.2%, and 3.1%, respectively. The absorption rate of 4.26 μm is the highest, and the energy of infrared radiation absorbed by CO2 gas at a specific wavelength (4.26 μm) is linearly related to the concentration of CO2 gas when the concentration of CO2 is low. That is, infrared light through the CO2 gas molecules, its radiation energy is reduced, the amount of infrared radiation energy absorbed with the gas absorption coefficient (K), gas concentration (c) and the thickness of the gas layer (L), and in line with the Lambert-Beer law, which can be expressed in the following formula:
E = E0KcL
Where: E0 an incident infrared radiation energy;
E a through the infrared radiation energy.
General infrared CO2 gas analyzer set up only to let the 4.26 μm infrared through the filter, the radiation energy that is E0, as long as the measured infrared radiation through the energy (E) the size of the gas, that is, we can know the concentration of CO2 gas .
Operation method
Determination of gas exchange in plant leaves by LI-6400 portable photosynthesizer
Principle
How the Infrared CO2 Gas Analyzer (IRGA) works. Infrared is an electromagnetic wave with a wavelength in the range of 0.75~400 μm. Infrared rays are classified according to their wavelength, 25~400 μm for far infrared rays, 2.5~25 μm for mid-infrared rays, and 0.75~2.5 μm for near infrared rays. The heated object is an excellent source of infrared radiation. Infrared radiation in the propagation of its radiant energy is absorbed by the object is easy to detect, this feature has become the basis for the design and manufacture of infrared CO2 gas analyzers. Different gases absorb infrared radiation differently. Gas molecules composed of the same kind of atoms (such as N2, H2, O2, etc.) do not absorb infrared. Only by the heterogeneous atomic composition of the gas molecules (such as CO, CO2, CH2, H2O, etc.) can absorb infrared. Because the gas molecules composed of heterogeneous atoms are permanent polar molecules, i.e. dipoles. The positions of the atoms within the molecule are in constant motion and undergo periodic changes. Under the action of infrared radiation of the same frequency, the dipoles (e.g., CO2, CH4) will resonate and absorb infrared radiation energy.When CO2 gas absorbs infrared radiation energy, its molecular structure will be changed from symmetrical type to telescopic or curved type. In addition, CO2 gas can absorb infrared energy in four bands, and the wavelengths of the absorption peaks are 2.66 μm, 2.77 μm, 4.26 μm, and 14.99 μm, and their absorption rates are 0.54%, 0.31%, 23.2%, and 3.1%, respectively. The peak value of 4. 26 μm has the highest absorption rate, and the infrared radiation energy absorbed by CO2 gas at a specific wavelength (4. 26 moan) is linearly related to the concentration of CO2 gas when the CO2 concentration is low. That is, infrared through the CO2 gas molecules, its radiation energy is reduced, was absorbed by the amount of infrared radiation energy and the gas absorption coefficient (K), gas concentration (c) and the thickness of the gas layer (L), and in line with the law of Lambert - Beer, which can be expressed in the following formula: E = E0KcL where: E0 an incident infrared radiation energy; E an infrared radiation through the energy. General infrared CO2 gas analyzer set up only to let the 4.26 μm infrared through the filter, the radiation energy that is E0, as long as the infrared radiation through the energy (E) the size of the measurement of the size of the radiation, that is, you can know the CO2 gas concentration.
Materials and Instruments
Material: leaves or plants with branches. Move The basic process of LI-6400 Portable Photosynthesizer to determine the gas exchange of plant leaves can be divided into the following steps: 1. Installation of the instrument: choose different leaf chambers for installation according to the object to be measured, and use the 6400-02B red and blue light source leaf chamber which is matched with the instrument for this experiment. Power supply line and the controller is correctly matched (pipeline and line must not be connected to the wrong), multi-hole plug line and analyzer alignment (red dot) inserted; hard plastic tube with a black ferrule on the end of the analyzer and the other end of the analyzer and the controller "sample" connected. Connect the air inlet pipe with "buffer", and connect the power supply (remember, except for the "sleep" state, do not connect or remove the pipeline and wiring when the power supply is turned on, otherwise the instrument will be burned). 2. Turn on the power: Turn on the power switch located on the right side of the main unit. The instrument will display "Is the IRGA connected?(Y/N)" after startup, select "Y"; select the leaf chamber (6400-02B red and blue light source), then enter <enter>, the instrument will display "Is the chamber connected? Display "Is the chamber/IRGA connected?(Y/N)", select "Y", the CO2 analyzer will have "Poof.... The CO2 analyzer will make a "poof..." sound and enter the main menu automatically. 3. 3. Manual Measurement: Press F4 "New Measurements" menu to enter the measurement menu. ①Parameter setting: Press 2, press F2 (Flow) to set the value of 100~500 to control the relative humidity inside the leaf chamber appropriately, <enter>; press F5 (Lamp OFF) to select Quantum flux <enter>; according to the type of plant to select the saturated light intensity (500~1,500), <enter>, press 1. Press 1. ② Matching: Tighten the nuts on the upper end of the soda lime tube and the drying tube towards Bypass. Close the leaf chamber, if " △CO2 " is more than 0.5 or less than 0.5, press F5 "Match". ③ Setting file: Press F1 "Open Logfile" to create a new file. Enter the file name you set. When the prompt "Enter Remark" appears on the display, enter the required mark (in English, it is used to mark the sample site, plant species, sample number, etc.). Continue to enter, the file setting is finished. ④ Measurement: Select the plant leaf to be measured and clip the leaf. Try to let the leaves fill the whole leaf chamber space, the area is 6 cm2, small leaves need to measure the area, and in the measurement menu state, press the number "3" and then press F1 to modify the value of the leaf area, close the leaf chamber, and tighten the fixing screws to the appropriate position. Wait for the PHOTO reading in row C to stabilize (the fluctuation of the last digit after the decimal point is about 2) and then you can record it (press the F1 "LOG" button or press the black button on the analyzer handle for 2 s to record one set of data). The best time for measurement is between 10:00 and 11:30 a.m. on a sunny day. ⑤ Environmental control: You only need to press the numbers to switch menus during manual measurement. ⑤ Environment control: You only need to press numbers to switch menus during manual measurement, as shown in Fig. 18-1. Temperature control: Press number 2, press F4 to control the temperature (input the desired temperature and enter, note that the temperature control range is ± 6 ℃ of the ambient temperature). Light intensity control: This function is used under the condition of connecting 6400-02B red and blue light source. In the measurement menu, press the number "2", press F5 to select "Quantum Flux" and enter, enter the desired light intensity value. CO2 Control: The 6400-01 CO2 injection system needs to be connected and the calibration menu can be accessed by selecting the F3 "Calib" button under the main menu. Close and tighten the leaf chamber and screw the CO2 filter tube to "SCRUB". Use the up and down arrows to select " CO2 Mixer Calibrate", enter, wait for the system to calibrate automatically, then return to the measurement menu, press number 2, press F3 to set the REF CO2 concentration can be measured. After the measurement is completed, turn off the airflow, temperature, light intensity control, back to the main menu. 4. Automatic measurement. (1) Light response curve: ① Create a file: clip on the leaf after the stomatal opening, after the establishment of a new file (under the Measurement menu, press number 1, press F1), match (under the Measurement menu, press number 1, press F5). ② Setting conditions: CO2 concentration, temperature, relative humidity. ③ Press the number 5 under the Measurement menu, press F1, select LIGHT CURVE, enter, and enter the light intensity gradient (e.g. 2000, 1500, 1200, 1000, 800, 600, 400, 200, 100, 50, 20, 0). Further enter the minimum wait time (e.g. 120 s) and the maximum wait time (240 s). Enter a matching value (e.g. 20 ppm), enter and the machine enters the automatic measurement, closes the file after the measurement and exits. (Note that this measurement requires little change in CO2 concentration, otherwise the concentration should be controlled.) (2) ACI curve: set the light intensity for the saturated light, other than the same as the light curve, the difference is that the choice of "ACI CURVE", set the concentration gradient (for example, 400, 300, 200, 100, 50, 400, 400, 600, 800, 1,000, 1,200, 1,500, 2,000). ). 5. Data output: connect the computer with the instrument, adjust the status of the instrument (under the main menu, press F5 "UTILITY" to enter the application menu, select "FILE EXCHANGE", and then enter). Run WINFX software, select CONNECT, and drag the data files under the "USER" folder in LI-6400 to a folder in your computer. Open the file with Excel software, select all files for the file extension, select the comma as the separator, and open the file to use the data. 6. 6. Shutdown: Press the "ESCAPE" button to return to the main menu, loosen the leaf chamber (leaving a small gap), the two chemical pipe nuts screwed to the middle of the slack state, turn off the host. Remove the battery to charge (Note: If the power is insufficient during use, the instrument will show sound prompts and text prompts, and the battery needs to be replaced. (When replacing the batteries, one battery should be replaced first and then the other).
Reagents:
① Anhydrous calcium chloride (anhydrous calcium sulfate)
② Caustic asbestos (10 mesh) or soda lime
Equipment: LI-6400 Portable Photosynthesis System.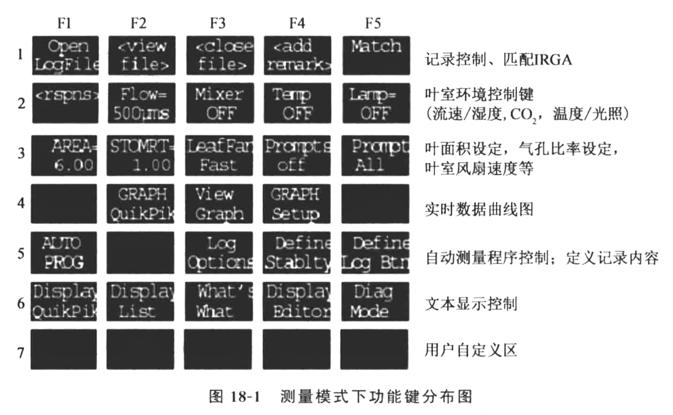
Ftime: duration (s); Photo: photosynthesis rate ( μmol・m-1・s-1 ); Cond: stomatal conductance (mmol H2O・m-2・s-1 ); Ci: inter-cellular CO2 concentration ( μmol・mol-1 ); Trmmol: transpiration rate ( mmol・m-2・s-1 ); VpdL: differential pressure of water and gas ( mg・L-1 ); Area: leaf area (mg・L-1); VpdL: leaf area ( mg・L-1 ); VpdL: water-air pressure difference ( mg・L-1 ); VpdL: water-air pressure difference ( mg・L-1 ); Area: leaf area (mg・L-1) ); Area: leaf area ( cm2 ); BLCond: interfacial layer conductance; Tleaf: leaf temperature (℃); St-mRat: stomatal ratio; Tair: air temperature (℃); TBlk: reference chamber (℃); CO2S: CO2 concentration in the leaf chamber ( μmol・mol-1 ); CO2R: CO2 concentration in the reference chamber ( μmol・mol-1 ); H2OR: water content in the reference chamber; H2OS: water content in the leaf chamber; RH_R: relative humidity in the reference chamber (%); RH_S: relative humidity in the leaf chamber (%); Flow: flow rate ( mL・s-1 ); CsMch: CO2S matching; PARi: light intensity inside the leaf chamber ( μmol・m-2・s-1 ); Press: atmospheric pressure (mPa); PARo: light intensity outside the leaf chamber ( μmol・m-2・s-1 ); PARo: light intensity outside the leaf chamber ( μmol・m-2・s-1 ); PARo: light intensity outside the leaf chamber (μmol・m-2・s-1) PARo: leaf outdoor light intensity ( μmol・m-2・s-1 ); HsMch: H2OS matching.
(iii) Content of experimental measurements1. Select one C3 and one C4 plant, and compare the differences in gas exchange parameters such as photosynthetic rate under saturated light between the two plants. 2.
2. Experimental data recording and processing:
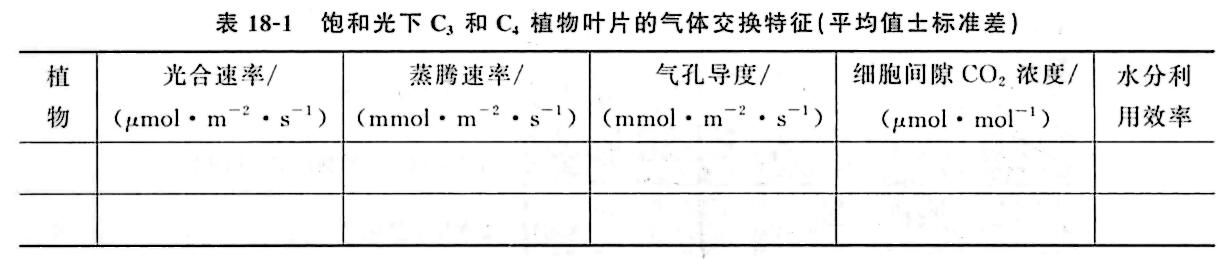
① Select and calculate the contents of Table 18-1 from the data recorded by the instrument.
② Select 2-3 groups of data from the existing class data and make a light-photosynthesis rate response curve that meets the requirements. Compare the differences between the response curves of the two plants.
③ Determine the light compensation point, saturation point, quantum efficiency and dark respiration rate of the two plants, and express them in a suitable way.
(iv) Analyze the differences in photosynthetic characteristics between C3 and C4 plants based on the results.
Caveat
1. The most basic requirement of the airtight system is that it is strictly airtight and cannot leak, otherwise it cannot be measured.
2. The filtering effect of the infrared instrument is not very satisfactory, and water vapor is the main factor interfering with the determination, therefore, the CaCl in the drying tube of the sampler2should be replaced frequently to avoid water absorption and dissolution into the infrared instrument, contamination of the analytical gas chamber to ensure measurement accuracy and extend the life of the instrument.
For more product details, please visit Aladdin Scientific website.
