2,5-di-tert-butyl-1,4-benzoquinone (2,5-DTBQ)

Product Manager:Nick Wilde
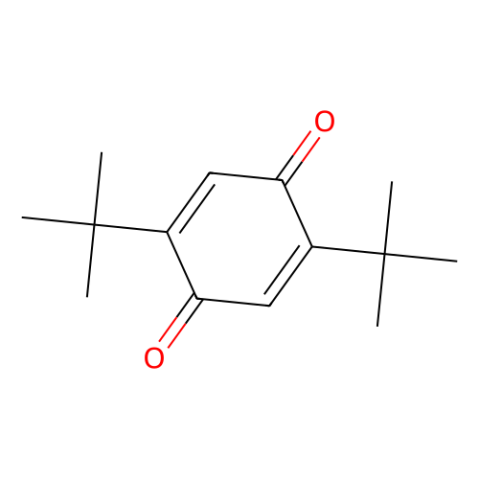
2,5-di-tert-butyl-1,4-benzoquinone (2,5-DTBQ)
See also:1,4-Benzoquinone
Recent Literature
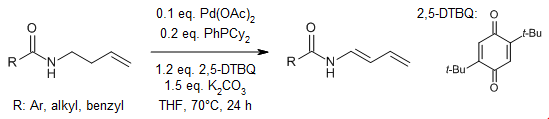
A palladium-catalyzed oxidative N-α,β-dehydrogenation of amides yields a variety of enamides in high yields, demonstrating excellent tolerance to various functional groups. This process entails the activation of allylic C(sp3)-H bonds, subsequently followed by β-H elimination.
Y.Jin, M. Li, Y. Chen, J. Li, W. Wu, H. Jiang, Org. Lett., 2024, 26, 4218-4223.
https://doi.org/10.1021/acs.orglett.4c01052
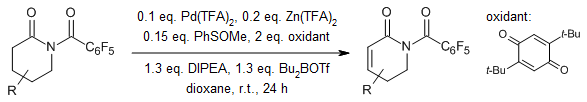
A Pd-catalyzed α,β-desaturation of N-protected lactams occurs under mild acidic conditions at room temperature, yielding their conjugated unsaturated derivatives. This reaction demonstrates broad functional group tolerance and offers reactivity distinct from previous desaturation techniques. Lactams featuring diverse ring sizes and substituents positioned at various points undergo smooth transformation.
M.Chen. G. Dong, J. Am. Chem. Soc., 2017, 139, 7757-7760.
https://doi.org/10.1021/jacs.7b04722
Quoted from:
https://www.organic-chemistry.org/chemicals/oxidations/2,5-di-tert-butyl-1,4-benzoquinone.shtm
Aladdin:https://www.aladdinsci.com
