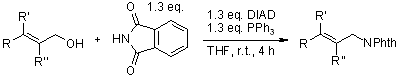
Diisopropyl azodicarboxylate (DIAD)
Product Manager:Nick Wilde
_%E8%8B%B1.png?access_token=43616dce-405f-47a1-954b-58cb85851142)
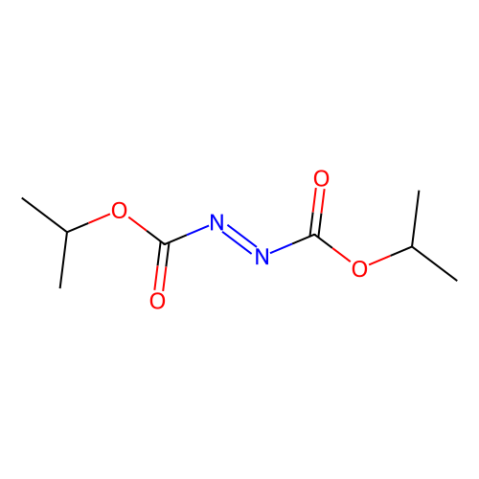
Name Reactions
Recent Literature
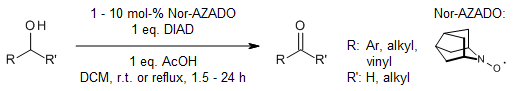
Using diisopropyl azodicarboxylate (DIAD) as a catalyst in a nitroxyl-radical-catalyzed oxidation process enables the transformation of various primary and secondary alcohols into their respective aldehydes and ketones, without progressing to overoxidation into carboxylic acids. Depending on the quantity of DIAD employed, 1,2-diols can be oxidized to either hydroxyl ketones or diketones.
M. Hayashi, M. Shibuay, Y. Iwabuchi, J. Org. Chem., 2012, 77, 3005-3009.
https://doi.org/10.1021/jo202588j
A photoorganocatalytic reaction between aldehydes and diisopropyl azodicarboxylate generates an intermediate carbonyl imide. This imide can then react with various amines to produce amides. This process allows for a gentle, single-step, and eco-friendly synthesis of amides from aldehydes and amines.
G. N. Papadopoulos, C. G. Kokotos, J. Org. Chem., 2016, 81, 7023-7028.
https://doi.org/10.1021/acs.joc.6b00488
A variant of the Larock method facilitates a streamlined synthesis of substituted quinolines in a single pot. This is achieved through a Heck reaction involving 2-bromoanilines and allylic alcohols, followed by dehydrogenation using diisopropyl azodicarboxylate (DIAD).
M. T. Stone, Org. Lett., 2011, 13, 2326-2329.
https://doi.org/10.1021/ol200579a
Quoted from:https://www.organic-chemistry.org/chemicals/oxidations/diisopropyl-azodicarboxylate-diad.shtm
Aladdin:https://www.aladdinsci.com