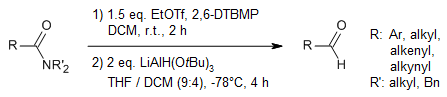Lithium tri-tert-butoxyaluminum hydride, LTBA

Product Manager
Sandra Forbes

Lithium tri-tert-butoxyaluminum hydride
Recent Literature
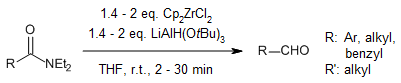
The in situ generation of the Schwartz reagent, which is highly efficient, offers a practical approach for reducing amides to aldehydes and achieving regioselective hydrozirconation-iodination of alkynes and alkenes. These one-step reactions are characterized by extremely brief reaction times, remarkable compatibility with functional groups, and the utilization of cost-effective and long-lasting reducing agents.
Y. Zhao, V. Snieckus, Org. Lett., 2014, 16, 390-393.
DOI: 10.1021/ol403183a
In a streamlined, single-step procedure for transforming sterically hindered N,N-diisopropylamides into aldehydes, the amides are first activated using EtOTf to produce imidates. These imidates are then reduced with LiAlH(OR)3, where R can be either t-Bu or Et, leading to the formation of hemiaminals. Subsequent hydrolysis of these hemiaminals yields the desired aldehydes. Notably, the inclusion of the non-nucleophilic base 2,6-DTBMP significantly enhances the efficiency of the reaction. This process is versatile and compatible with a range of reducible functional groups, such as aldehydes and ketones.
P. Xiao, Z. Tang, K. Wang, H. Chen, Q. Guo, Y. Chu, L. Gao, Z. Song, J. Org. Chem., 2018, 83, 1687-1700.
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/lithium-tri-tert-butoxyaluminum-hydride-ltba.shtm
Aladdinsci: https://www.aladdinsci.com