Dendrons and Hyperbranched Polymers: Multifunctional Tools for Materials Chemistry

Product Manager
Sandra Forbes
Introduction
Dendrimers are polymeric macromolecules constructed from multiple monomers that are perfectly branched and radiate outward from a central core (Figure 1). As one moves from the dendrimer's core towards its surface, the number of branch points increases, which determines the generation of the dendrimer. The branched structure provides dendrimers with several distinctive properties, making them suitable for various material applications.
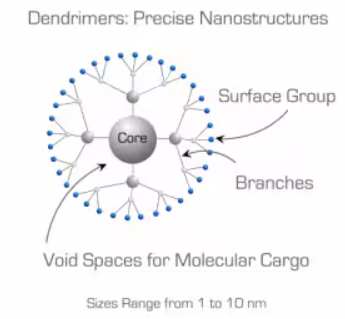
Figure 1. Schematic representation if a generation 2 dendrimer.
· The compact nm-scale structure of dendrimers leads to high solubility and low viscosity in solution, making them suitable for applications as rheology (viscosity) modifiers.1
· The dendrimer surface exhibits a dense array of multiple terminal groups (multivalency), with the number of these surface groups increasing with each generation of dendrimers. This multivalency can be utilized to achieve high concentrations of chemically grafted drugs or imaging labels on the dendrimer surface.2
· The dendrimer features a core-shell molecular architecture that possesses a chemically distinct interior and surface. This architecture allows for the encapsulation and controlled release of molecules that are chemically incompatible with the external environment, such as catalysts, drugs, or chromophores.3
Aladdin provides a diverse selection of monodisperse dendrimer molecules featuring four distinct chemical structures (PAMAM, phosphorus-based, polypropylenimine, and polylysine) along with numerous unique terminal surface groups. To broaden the spectrum of dendritic tools available for your materials chemistry research, we are delighted to introduce two additional types of dendritic macromolecules: polyester dendrons and hyperbranched polymers constructed from the 2,2-bis(hydroxymethyl)propanoic acid (bis-MPA) monomer.
Dendrons
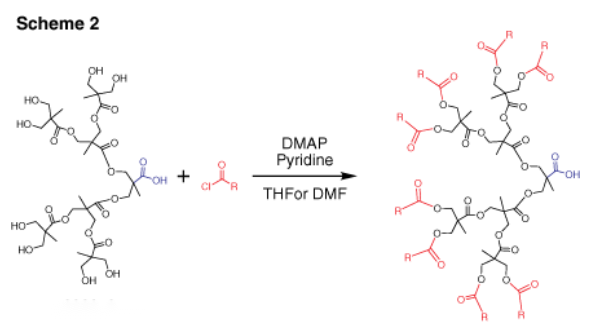
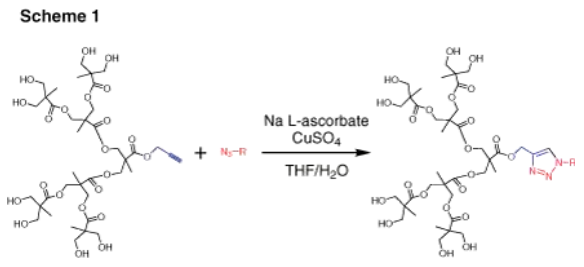
Dendrons (depicted in Figure 2) represent monodisperse, wedge-shaped sections of dendrimers, characterized by multiple terminal groups and a single reactive function at their focal point. This unique configuration, combined with the properties mentioned earlier, offers the flexibility to perform orthogonal reactions by leveraging both the distinct focal point and surface groups. For instance, dendrons can be grafted onto surfaces, to other dendrons,4 or to other macromolecules.5 Our bis-MPA dendron offerings span three generations, featuring either alkyne or carboxylic acid focal point functionalities. Alkyne functions are ideal for "click chemistry" via catalytic cycloaddition with azides (as illustrated in Scheme 1). Carboxylic acids, on the other hand, can undergo selective reactions with amines, typically facilitated by EDC-mediated coupling. Furthermore, the multiple surface hydroxyl groups can be further functionalized through reactions with anhydrides or acyl halides (as shown in Scheme 2).
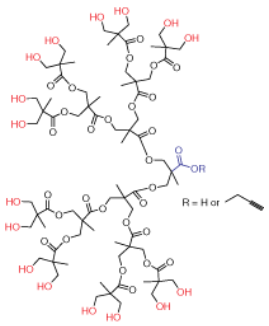
Figure 2. Generation 4 bis-MPA dendron
Hyperbranched Polymers
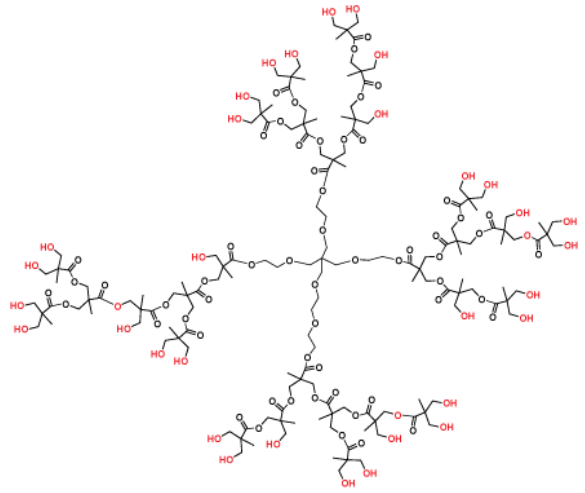
Hyperbranched polymers (depicted in Figure 3) are polydisperse dendritic macromolecules that exhibit dendrimer-like characteristics yet are synthesized through a single polymerization step. These polymers feature imperfect branching and possess an average (rather than a precise) count of terminal functional groups. Despite their imperfect structure, hyperbranched polymers offer a more cost-effective alternative compared to perfectly branched dendrimer products.
Figure 3. Hyperbranched bis-MPA polyester Generation 3
For applications that can accommodate imperfections, hyperbranched polymers offer the benefits of dendrimers at a significantly reduced cost. Our bis-MPA hyperbranched polymer products are of high purity (>97% purity) and are provided in a lyophilized powder form that is easy to dissolve.
Features and Benefits
The distinctive features and advantages of our new hyperbranched polymer products encompass:
· An abundance of hydroxyl (-OH) end-groups, readily accessible for further chemical functionalization through reactions with anhydrides and acyl halides, among others.
· Versatility as precursors for hybrid dendritic-linear materials.6
· A high degree of branching that enhances solubility and reduces viscosity (both in solution and in the melt state).
· Utility as multifunctional initiators and cross-linkers in ring-opening polymerizations and thermoset formulations.
· Optical transparency within the ultraviolet-visible wavelength range.
Both polyester dendrons and hyperbranched polymers are soluble in water and polar organic solvents such as methanol, THF, DMF, and DMSO. Studies have shown that bis-MPA dendritic macromolecules exhibit biocompatibility and biodegradability, with non-toxic degradation products. These attributes render them highly suitable for applications in drug delivery and other biomaterial contexts.7
References
1. Dvornic P, Uppuluri S. 2002. In Dendrimers and Other Dendritic Polymers. John Wiley & Sons, Ltd.
2. Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. 2005. Designing dendrimers for biological applications. Nat Biotechnol. 23(12): 1517-1526. https://doi.org/10.1038/nbt1171
3. Svenson S, Chouhan A, Reyna L, Tomalia D. 2007. Solubility enhancement of poorly water soluble molecules using Dendrimers.. Material Matters. 224-26..
4. Wu P, Malkoch M, Hunt JN, Vestberg R, Kaltgrad E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. 2005. Multivalent, bifunctional dendrimers prepared by click chemistry. Chem. Commun..(46): 5775. https://doi.org/10.1039/b512021g
5. Gillies ER, Fréchet JMJ. 2002. Designing Macromolecules for Therapeutic Applications: Polyester DendrimerPoly(ethylene oxide) “Bow-Tie” Hybrids with Tunable Molecular Weight and Architecture. J. Am. Chem. Soc.. 124(47): 14137-14146. https://doi.org/10.1021/ja028100n
6. Arce E, Nieto PM, Díaz V, Castro RG, Bernad A, Rojo J. 2003. Glycodendritic Structures Based on Boltorn Hyperbranched Polymers and Their Interactions withLens culinarisLectin. Bioconjugate Chem.. 14(4): 817-823. https://doi.org/10.1021/bc034008k
7. Padilla De Jesús OL, Ihre HR, Gagne L, Fréchet JMJ, Szoka FC. 2002. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and In Vivo Evaluation. Bioconjugate Chem.. 13(3): 453-461. https://doi.org/10.1021/bc010103m
Aladdinsci: https://www.aladdinsci.com